References
Nemaline myopathy and severe dentofacial deformity − a case report
From Volume 11, Issue 2, April 2018 | Pages 67-73
Article

Congenital myopathies are a group of clinically and genetically heterogeneous neuromuscular disorders that present with muscle weakness and hypotonia, particularly relating to the proximal muscles. The incidence of these disorders is estimated at 6 per 100000 live births.1 Diagnosis is normally made in childhood after observation of floppy infants that fail to reach motor milestones.2 Occasionally, adult onset forms have been reported. As well as histological and clinical findings, MRI muscle imaging and genetic screening can be used to differentiate between myopathy types. Sufferers normally experience muscle weakness, muscle slowness and hypotonia and have reduced muscle mass. Lordosis (excessive inward curvature of the lower back) and a long face with a high arched palate are also characteristic of the disorder.3,4 Scoliosis has also been observed, although this may be due to patients being wheelchair bound during puberty rather than the true disease process. Patients diagnosed with congenital myopathies are at risk of premature death from respiratory failure and occasionally cardiac problems. A minority of patients have also been found to suffer mental retardation.5
Nemaline myopathy is the most common non-dystrophic congenital myopathy, with an incidence of 2 in 100000 live births. It is a slowly developing or non-progressive type of myopathy diagnosed histochemically or with electron microscopy of muscle biopsies. A positive diagnosis can be made by identifying the presence of nemaline rods. These are thread-like bodies clustered under the sarcolemmal membrane, around or (rarely) within the nuclei of skeletal muscle cells.6 Areas with extensive rod formation exhibit significant disruption and disorganization of the sarcomeric structure that may account for some of the associated muscle weakness. Case reports suggest that nemaline myopathy patients have the classic long face and high arched palate typical of the facial features of other congenital myopathies.7,8
There are seven genotypically distinct types of nemaline myopathy (Table 1).9 The ENMC International Consortium on Nemaline Myopathy defined six clinical categories of nemaline myopathy based on knowledge of the typical form and how other groups of patients differ from this (Table 2).10 Diagnosis is not always clear cut, with considerable overlap of clinical presentation for the genetically distinct subgroups.
| NEM1 | Slow a-Tropomyosin Gene | TPM3 |
| NEM2 | Nebulin Gene | NEB |
| NEM3 | Skeletal Muscle a-Actine Gene | ACTA1 |
| NEM4 | b-Tropomyosin Gene | TPM2 |
| NEM5 | Slow Skeletal Muscle Troponin T Gene | TNNT1 |
| NEM6 | Kelch Repeat and BTB (POZ) Domain Containing 13 | KBTBD13 |
| NEM7 | Skeletal Muscle Cofilin-2 Gene | CFL2 |
| 1 | The severe congenital form, with patients lacking spontaneous movements or respiration at birth, or with fractures or severe contractures at birth |
| 2 | The intermediate congenital form, with patients moving and breathing at birth but who are later unable to achieve ambulation or respiratory independence |
| 3 | The typical congenital form, with typical distribution of muscle weakness, milestones delayed but reached, and slowly progressive or non-progressive course |
| 4 | Mild nemaline myopathy with childhood onset |
| 5 | Adult onset nemaline myopathy |
| 6 | Other forms of nemaline myopathy with unusual associated features |
Cardiac involvement has been reported in nemaline myopathy patients. It can be fatal due to ventricluar failure, with postmortem histological findings demonstrating nemaline bodies found in the myocardium, the conducting tissue of the heart, as well as the skeletal muscle.11
There is currently no effective treatment for nemaline myopathy. Symptomatic treatment includes: ventilatory support for breathing disorders, nasogastric feeding and, in the milder forms, orthoses. Genetic counselling enables families to make informed decisions regarding subsequent pregnancies. Dietary L− Tyrosine supplements are currently being investigated along with exercise therapy. The drug Ataluren is currently being trialled on patients with Duchenne Muscular Dystrophy (DMD) to cause read-through of targeted stop codons and could theoretically be tested on certain genetic variants of nemaline myopathy. Gene replacement or up-regulation therapy could play a role in the future, along with stem cell therapy for adult onset nemaline myopathy.
Case report
Patient JD presented aged 14 years 8 months complaining of her dental appearance and function. She had no learning difficulties and had been diagnosed with nemaline myopathy. Patient JD was wheelchair bound, fed by Percutaneous Endoscopic Gastrostomy (PEG) and was receiving non-invasive ventilatory support for her night-time breathing disorder.
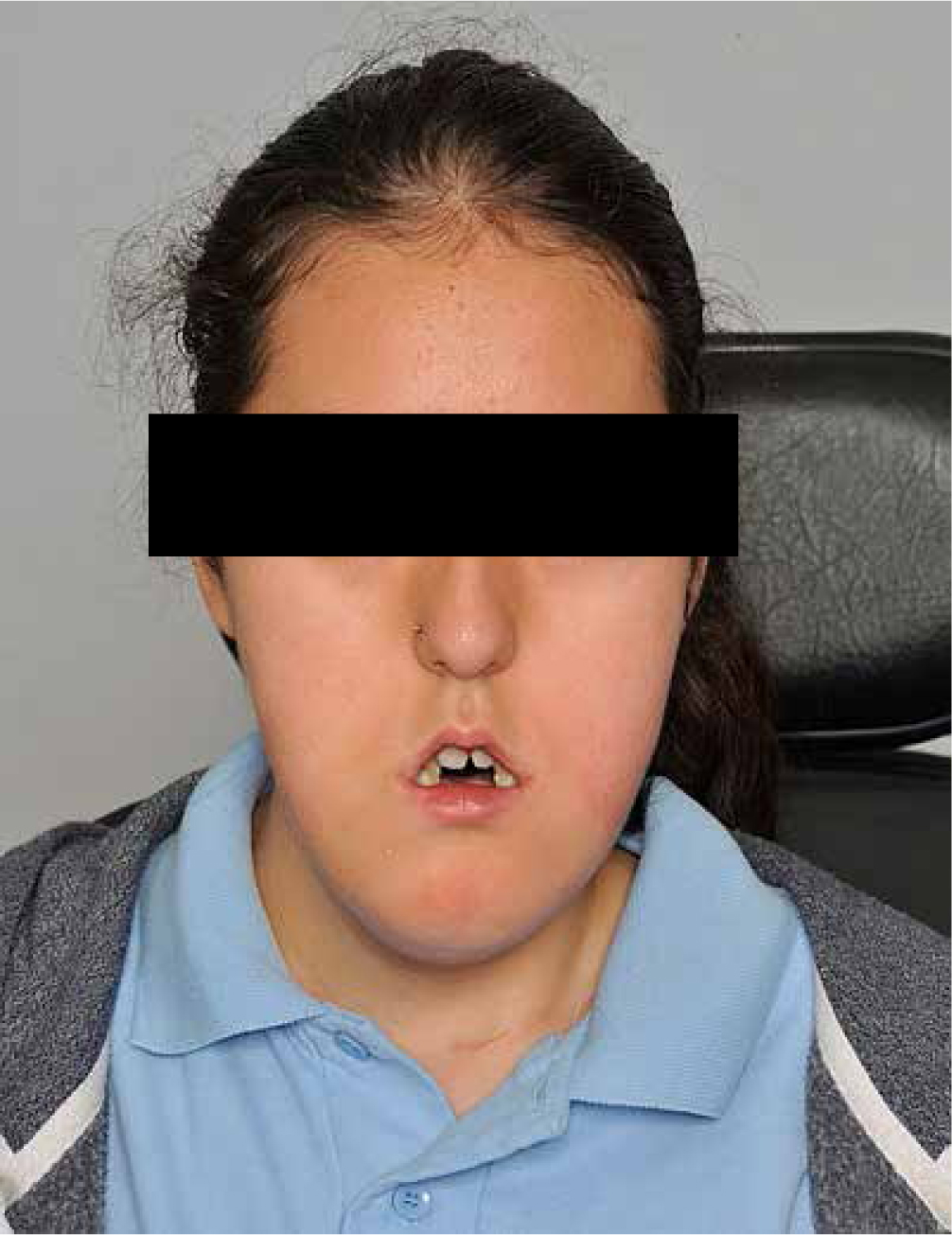
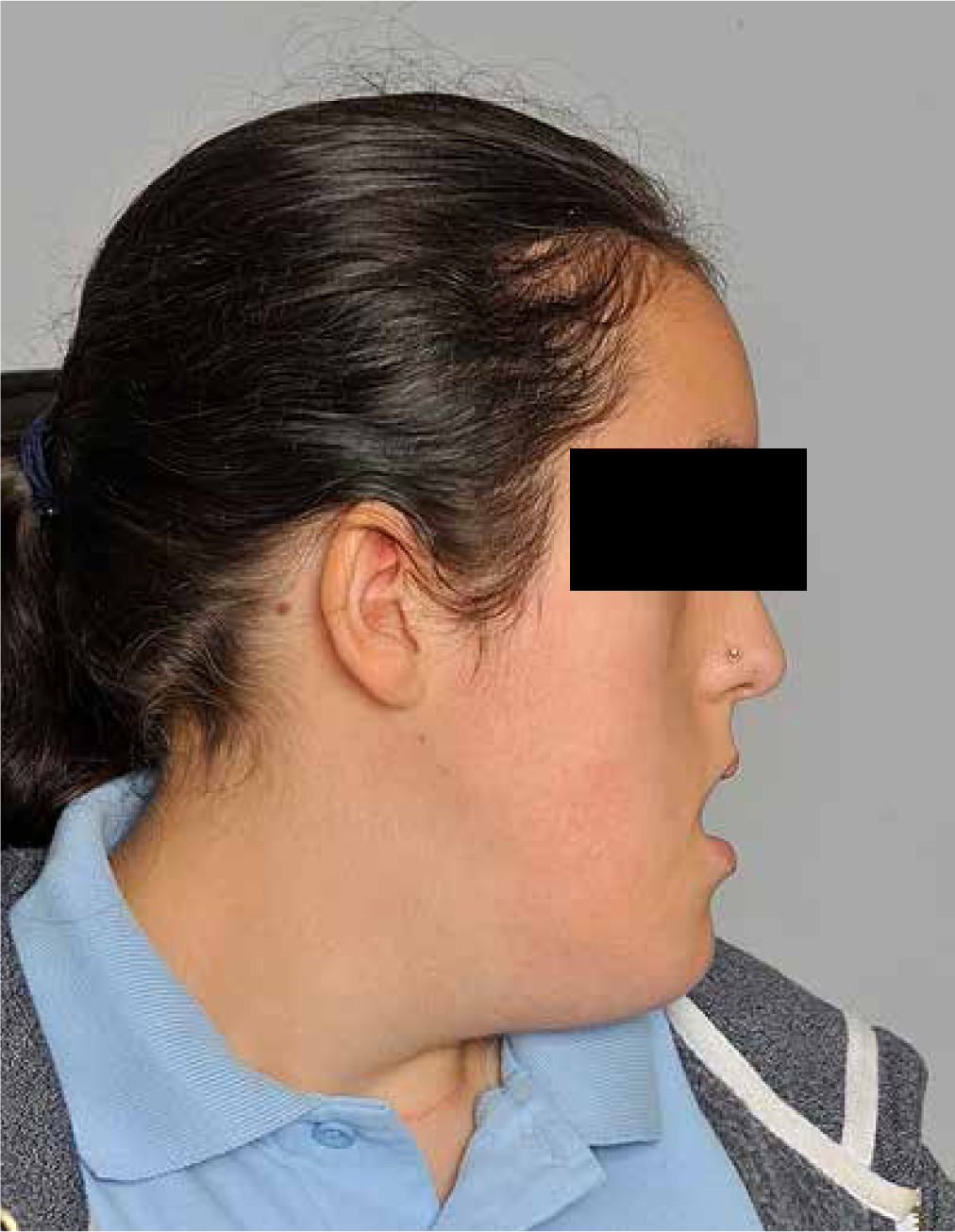
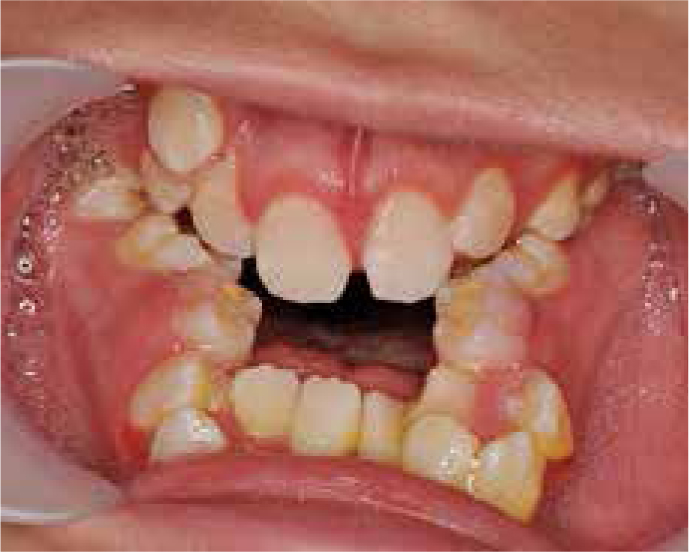
Clinical examination revealed a severe Class III skeletal pattern with midface retrusion, increased lower face height, increased gonial angle and mild mandibular asymmetry (Figures 1 and 2). Intra-oral examination revealed a Class III malocclusion with multiple retained deciduous teeth, delayed eruption of some permanent teeth, a 9 mm AOB and a 6 mm reverse overjet (Figure 3). Oral hygiene was poor, with generalized gingivitis and demineralization, but caries experience was low as a result of the PEG feeding. The upper arch was severely crowded and narrow with palatally inclined buccal segment teeth. There was an unerupted upper left canine and a buccally positioned upper right canine. The lower arch was also narrow with moderate crowding and lingually inclined posterior and anterior teeth. There was a unilateral crossbite affecting the right-hand side and centreline discrepancies of the upper and lower arches that were non-coincident. The palate was very deep and there was significant enlargement of the palatal mucosa.
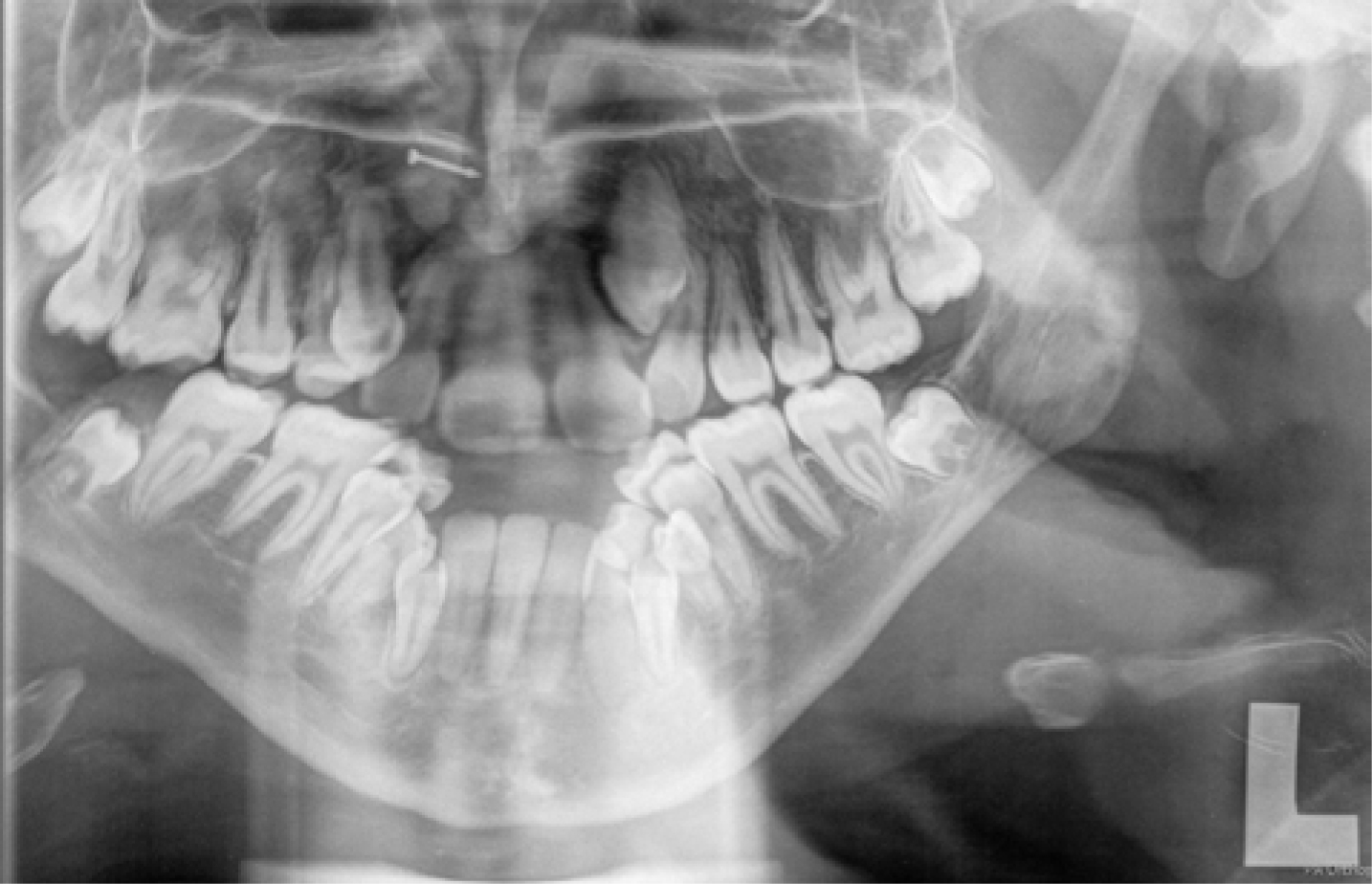
Positioning for panoramic radiography was difficult (Figure 4) but all the permanent teeth were identified as being present, including the third molars. Vertical parallax using a maxillary occlusal radiograph indicated the crown of the unerupted upper left canine tooth was buccally positioned. Cephalometric assessment (Figure 5 and Table 3) confirmed a high angle Class III skeletal pattern with significant maxillary retrognathia and increased lower anterior face height. Mandibular length was found to be average with maxillary length two standard deviations shorter than the norm.
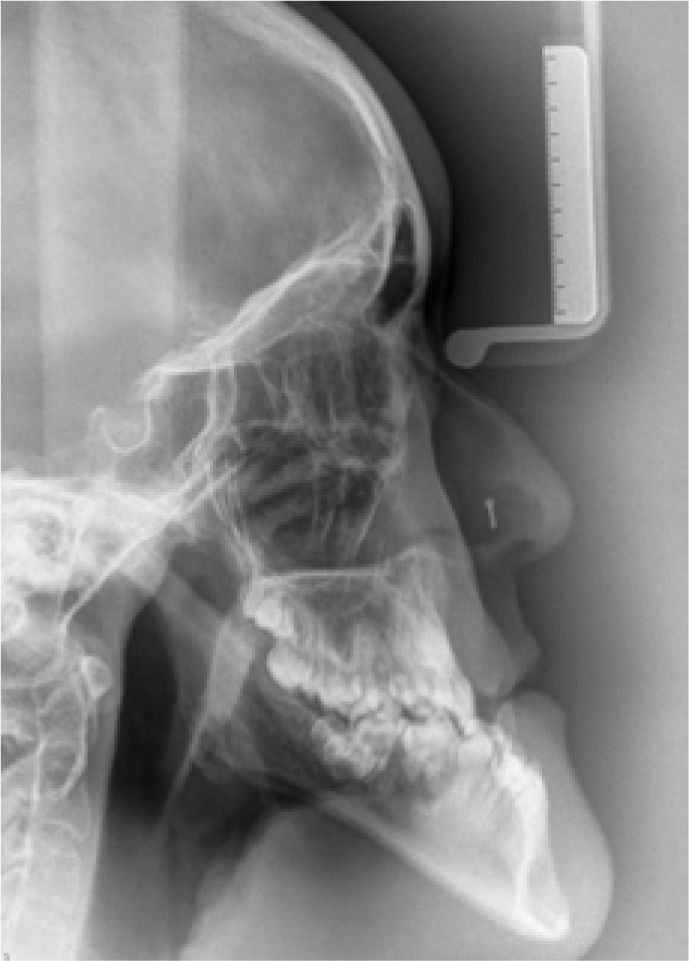
| SNA | 72.9° |
| SNA | 81.0° |
| ANB | -8.1° |
| SN:MxP | 18.0° |
| SN:MnP | 52.4° |
| MMPA | 34.4° |
| UIMxP | 107.7° |
| LIMnP | 63.5° |
| UAFH | 51.5 mm |
| LAFH | 75.7 mm |
| LAFH:TAFH | 59.5% |
| Co-Pog | 118.2 mm |
| Co-ANS | 69.6 mm |
| Pog-Nperp | 2.1 mm |
| A-Nperp | -7.5 mm |
Management options were discussed with the patient, including relief of crowding followed by monitoring, compromise orthodontics or possible orthognathic treatment. The patient was seen on an orthognathic clinic with her consultant in paediatric respiratory medicine. Her main desire was to be free of her night-time ventilatory support. When there was no guarantee that this would occur with orthognathic treatment, the patient opted for compromise alignment orthodontic treatment when the permanent teeth had fully erupted
Discussion
This case illustrates a severe malocclusion that can result when facial growth is adversely affected by a muscle weakness disorder. It is generally agreed that malocclusions are the result of a poorly understood and complex relationship between hereditary and environmental influences, with muscle dysfunction one of the known causes.12 Patients with anterior open bites have been demonstrated to have reduced bite forces but a cause and effect relationship has never been established.13 Patients with muscle weakness disorders do have more malocclusions, with increased vertical proportions and weaker bite forces than controls.14 Although the weak facial musculature is bound to be a significant factor in the aetiology of long face syndrome, it is more likely that it is the altered posture secondary to this that is causative.15 This is supported by the occurrence of long face syndrome in patients with other syndromes or anomalies, such as connective tissue disturbances and mouth breathing secondary to nasal obstruction.16,17,18
Patients with non-specific muscle weakness have historically been reported as having characteristically long faces and open bites.19 Muscle dysfunction can theoretically disturb facial growth by altering bone formation at the point of muscle insertion, leading to an underdeveloped and narrow mandible, as well as generalized under-development of the jaws leading to dental crowding. The other concomitant theory is that these muscle weakness disorders disrupt the soft tissue matrix that caries the jaws downwards and forwards. It is proposed that the low tongue and mandibular position cannot counterbalance the forces developed by the stretched facial musculature, decreasing the width of the palate. Overeruption of the posterior teeth caused by the low mandibular position leads to the anterior open bite.20 The increased gonial angle and increased face height suggest that an intra-matrix backward rotation occurs as part of the total mandibular growth rotation.21
Conditions with decreased muscle tone, such as muscular dystrophy, cerebral palsy and the congenital myopathies can result in significant malocclusions.22,23 There is weak evidence to suggest that malocclusions secondary to neurogenic muscle weakness disorders, such as cerebral palsy, are milder than those caused by genuine myopathies.24 The malocclusions appear to manifest before adolescence but, as age of onset of these conditions can vary, it would make sense that the severity of malocclusion would be worse in a patient affected during growth compared to a patient with adult onset disease.
A nemaline myopathy patient, with a moderate dentofacial deformity and severe anterior open bite, was reported as treated successfully with orthodontics and bimaxillary osteotomy surgery followed by reduction rhinoplasty and a genioplasty six months later.25 The occlusal result was reported to be stable 18 months post-operatively. A case report of a patient with myotonic dystrophy was also successfully treated with orthognathic surgery, but there was no long-term follow-up regarding stability and a delayed recovery from the anaesthetic and a prolonged hospital stay was reported.26 The stability of orthognathic surgery for long face syndrome patients has been questioned and should be especially considered by the clinical team when the cause of malocclusion persists after surgery in patients with a muscle weakness disorder.27
If a patient with nemaline myopathy is planned for elective surgery, such as an orthognathic patient, close liaison with anaesthetic colleagues is necessary. The anaesthetist will want to know the exact underlying molecular mechanism of the myopathy and the extent of airway and breathing problems.28 Although there are no specific reports of malignant hyperthermia in nemaline myopathy patients, they are treated with the same level of caution for this adverse event as those diagnosed with central core disease myopathy. It is highly likely that depolarizing muscle relaxants will be avoided during surgery and an in vitro contracture test for risk of malignant hyperthermia performed.29
Sleep disordered breathing can occur in patients with congenital myopathies and muscular dystrophies.30 This can be due to airflow limitation, hypoventilation, upper airway obstruction or central apnoea. These patients are commonly treated with one of the various forms of non-invasive ventilation systems, depending on the diagnosis of the sleep-disordered breathing condition.31 Sleep hypoventilation requiring overnight ventilatory support has been reported in a patient with nemaline myopathy.32 As suggested for patients with muscular dystrophy, investigation of sleep-related breathing disorders in patients with congenital myopathy can identify the underlying cause.33 If obstructive sleep apnoea is diagnosed and the obstruction is confirmed to be from the upper airway, then surgery could theoretically be considered. Caution should be taken prior to considering operating on nemaline myopathy patients as temporary central sleep apnoea can occur following nasal and maxillofacial surgery for obstructive airway conditions.34,35 As well as the previously discussed breathing disorders and possible anaesthetic risk, nemaline myopathy sufferers can develop fatal sudden onset respiratory failure.36 This can occur, without notice, in ambulant individuals with the milder forms of the disease.
In this case report, a severe malocclusion in an adolescent patient with nemaline myopathy who presented for orthodontic treatment to improve function and facial appearance has been reported. The anaesthetic risk, life expectancy and long-term stability of surgical procedures should be considered when managing a patient with a severe malocclusion due to a congenital muscle weakness disorder such as nemaline myopathy. Although this patient is unlikely to undertake orthognathic treatment, it is hoped that this case report will improve understanding of the condition and help other teams plan and consent patients with similar severe malocclusions and dentofacial discrepancies.
